Biomed Engineering Services
We deploy state of the art, mission-critical technologies in hospitals. We believe that quality assurance in our products and Biomed services is the key to our company’s success. We represent various European and U.S manufacturers and always ready for the latest cutting-edge technologies to advance and improve the health care providers.
We have been awarded by the Ministry of Health (MOH) in the UAE “The supplier of the year” award as recognition of our dedication, quality and service.
We are a single-source solution for Medical Equipment Technical Service and Support. Our medical sales and services offer an affordable way to meet your clinical equipment needs without compromising quality and patient safety.
Projects
Starts from the planning of equipment to disposal. Our trained biomed engineers can guide you in all these complex and stagnant processes effectively for any turnkey projects. We help answer questions and explain documented procedures during institutional /international equipment inspection audits. All our Biomedical services follow DHA/HAAD/MOH/DHCC policies
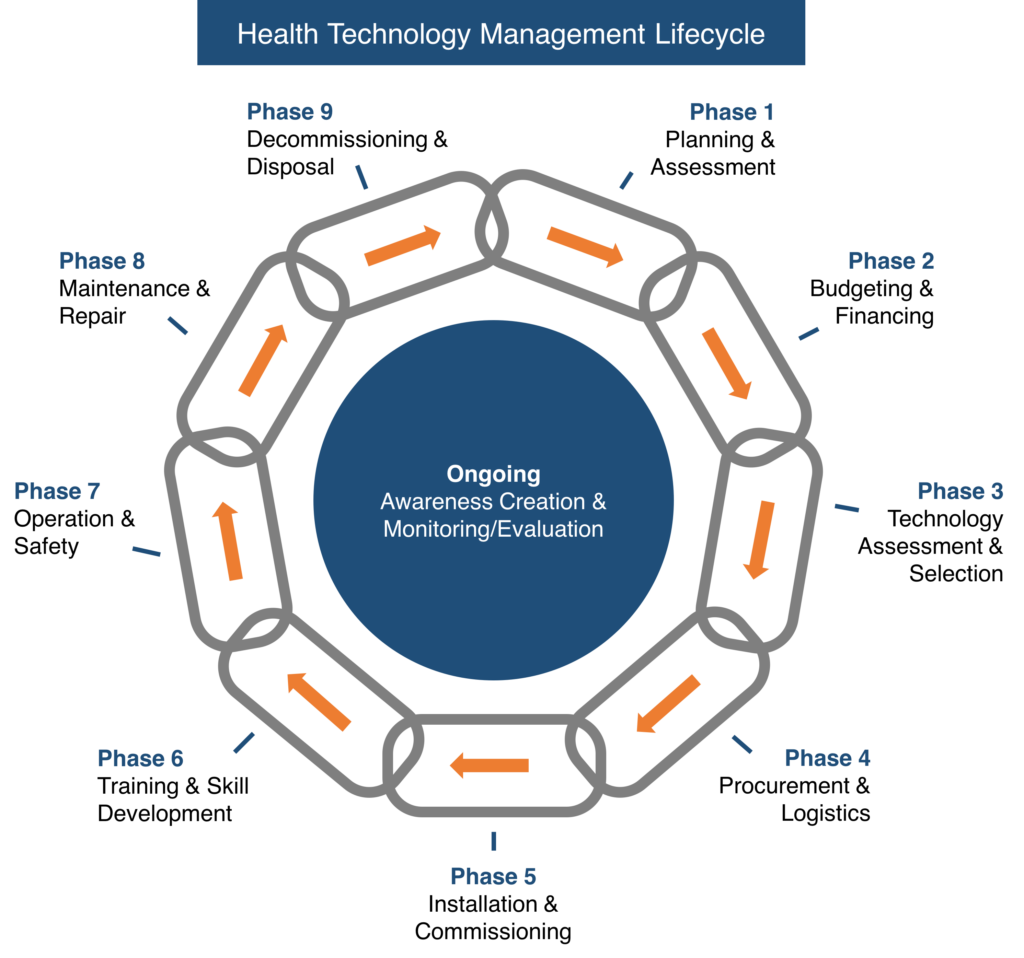
Health Technology Management Cycle
Our Biomedical Engineers will complete the following tasks after every installation:
- Installation report
- Installation checklist
- Warranty certificates
- Periodic Preventive Maintenance (PPM) schedule
- End-user Training
- Service Manual
- User Manual
- Required accessories list
- Required disposables list
- Test tool calibration report
- Engineer training
- EST report
Computerized asset management:
Our web-based & automated medical management software program designed specifically for Biomedical Equipment programs. The program will supply you with all the reports necessary to show your compliant, tracks all maintenance history, and provides a preventive maintenance schedule for our team to follow.
Preventive Maintenance contracts:
Preventive Maintenance programs are a key part of our services. Designing, implementing, and completing a service schedule is tedious and strains internal resources. Accurate and consistent readings rely on adequately serviced and calibrated medical equipment.
These services include:
- Test and calibrate all configurations of the device to the manufacturer’s specs.
- Electrical safety on all electrical equipment to ensure it is in compliance with standards for healthcare facilities.
- Each device will be assigned a control number for equipment tracking. Each control number will receive a printed document stating what tests were completed.
- Each PM service submission is with PPM checklist and report.
- Professional stickers placed on equipment stating due date.
- PM carried out following specific benchmark operating protocols compliant with the manufacturer’s instructions and local and international standards.
Consulting Services:
Consulting services which includes equipment purchasing; contract consolidation and assistance; service consultation regulatory compliance best practices; and in-services. We become true partners in technology management by providing a variety of consulting services including:
- Equipment purchasing consultation– based on your facility’s needs, equipment history, and end-user compatibility
- Contract consulting and consolidation– reducing costs and vendor management by consolidating contracts and/or modifying contract arrangements
- Regulatory compliance best practices– gained by years of experience helping health care organizations prepare to meet compliance standards
- In-services– initial or continuing education in-services for patient care equipment
- Targeted advice for any equipment replacement.
Corrective Service:
All of our repairs adhere to specific manufacturer’s recommendations. With maximum uptime. Relationships with major biomedical equipment manufacturers let us deliver a comprehensive service optimized provision of spare parts too.
User Training:
Our equipment training helps improve the safety and efficiency of medical equipment and deliver better patient care. All user will be awarded training completion certificates after successful completion of any particular equipment training.
Disposal/ Replacement:
We apply standardized procedures to evaluate the life of any equipment and recommendation for condemnation based on manufacturer’s recommendations, current standards, maintenance record, safety and quality of the equipment.
Please feel free to contact us to discuss your Biomed engineering services.